FDA 510(k)
Premarket Notification Clearance
Medical Device Clearance
The FDA 510(k) clearance is a specific premarket submission made to the FDA to demonstrate that our device is safe and effective—substantially equivalent to a legally marketed device that is not subject to premarket approval.
Verified device safety and efficacy
Substantial equivalence to legally marketed devices
FDA 510(k) Clearance
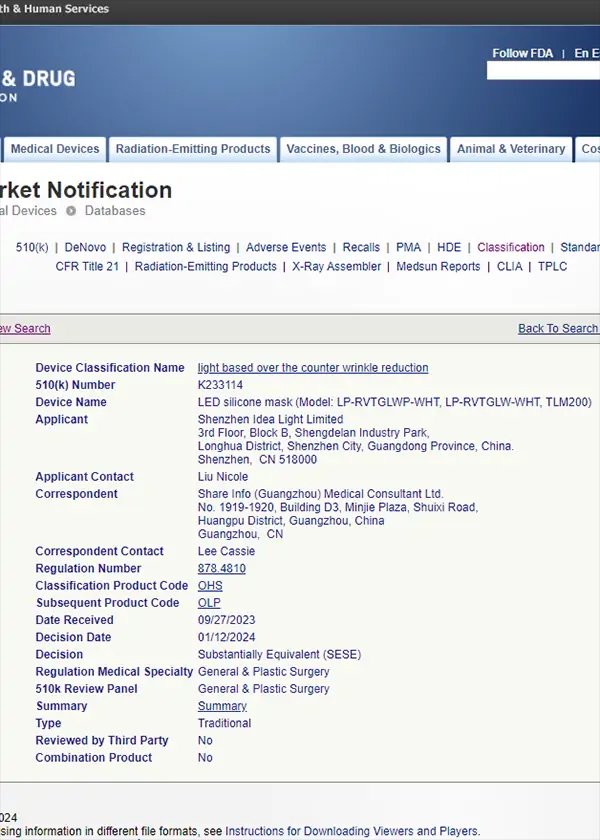
Click to view full certificate
Proven Performance
Our FDA 510(k) clearance is the result of extensive testing and validation. It confirms that our technology not only meets but exceeds the necessary requirements for safety and effectiveness, providing healthcare professionals and consumers with confidence in using our devices.
What FDA 510(k) Clearance Means for You
The FDA 510(k) clearance represents one of the highest standards of medical device regulation in the United States. For ErythrosLight customers, this certification provides several critical assurances about our SciLight Pro and Laser Pro Mask devices.
Unlike many red light therapy devices on the market that operate without regulatory oversight, our FDA clearance means we've undergone rigorous testing protocols. This includes electromagnetic compatibility testing, electrical safety evaluations, and clinical performance validation that demonstrates our devices deliver the therapeutic benefits we claim.
The 510(k) process requires us to prove "substantial equivalence" to previously cleared devices while demonstrating our innovations in wavelength optimization and power delivery. Our 9-wavelength technology underwent extensive review to ensure each wavelength's safety and efficacy for photobiomodulation therapy.
Clinical Applications and Benefits
Our FDA 510(k) clearance specifically covers therapeutic applications including muscle recovery, joint pain relief, and wound healing acceleration. This regulatory approval allows healthcare professionals to confidently recommend our devices for clinical use, making ErythrosLight a trusted choice for both home users and medical practices.
Cleared Indications:
- Temporary relief of minor muscle and joint aches
- Relaxation of muscle spasms
- Temporary increase in local blood circulation
- Muscle and joint stiffness relief
Why Choose FDA-Cleared Devices?
When investing in red light therapy, choosing an FDA-cleared device ensures you're getting technology that has been thoroughly vetted for safety and effectiveness. Learn more about our commitment to quality and how our certifications translate to better outcomes for users.
The FDA 510(k) process validates not just our devices, but our entire approach to photobiomodulation therapy. From our precision-engineered wavelengths to our advanced power delivery systems, every aspect has been scrutinized to meet medical device standards.
Note: FDA clearance demonstrates safety and effectiveness for the cleared indications. Individual results may vary. For specific medical conditions, consult with your healthcare provider about incorporating red light therapy into your treatment plan.